PPT Introduction to Mass Spectrometry PowerPoint Presentation, free download ID1273270
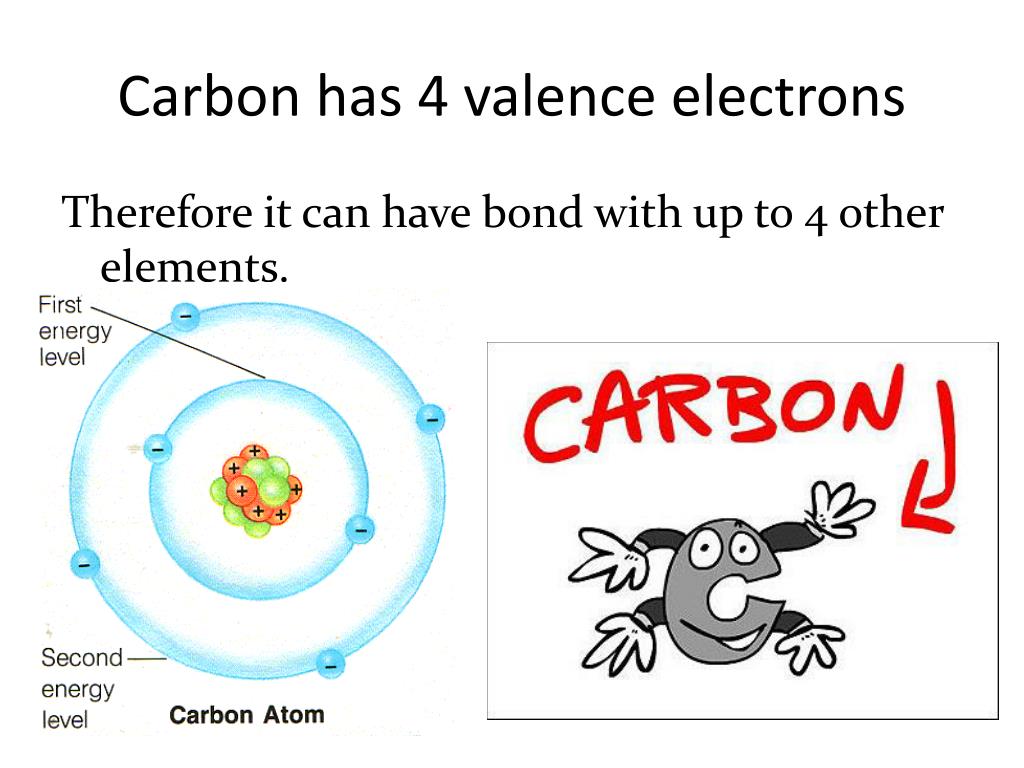
How many valence electrons does carbon have? Flexi Says: Carbon (C) is a nonmetal in group 14 of the periodic table. Like other group 14 elements, carbon has four valence electrons. Discuss further with Flexi.

Carbon — Role and Importance to Life Expii
About Transcript How to determine the number of valence electrons and draw Lewis structures for main group elements starting from the electron configuration. Created by Jay. Questions Tips & Thanks Want to join the conversation? Sort by: Top Voted Manahil Ahmed 9 years ago If helium is in group 8 why does it not have 8 valence electrons .

Periodic Table Carbon Periodic Table Timeline
1. Determine the total number of valence electrons in the molecule or ion. Each hydrogen atom (group 1) has one valence electron, carbon (group 14) has 4 valence electrons, and oxygen (group 16) has 6 valence electrons, for a total of [(2)(1) + 4 + 6] = 12 valence electrons. 2. Arrange the atoms to show specific connections.
Carbon Has Four Valence Electrons And The Lewis Structures Of Methane My XXX Hot Girl
Valence electrons: For main group elements (i.e s-block and p-block elements), the valence electrons are the electrons present in the outermost orbit. But for most of the transition and inner transition elements, the valence electrons are the electrons present in the shells outside the noble gas core.
What Are Valence Electrons / Valence Electrons / The nucleus is what contains the protons
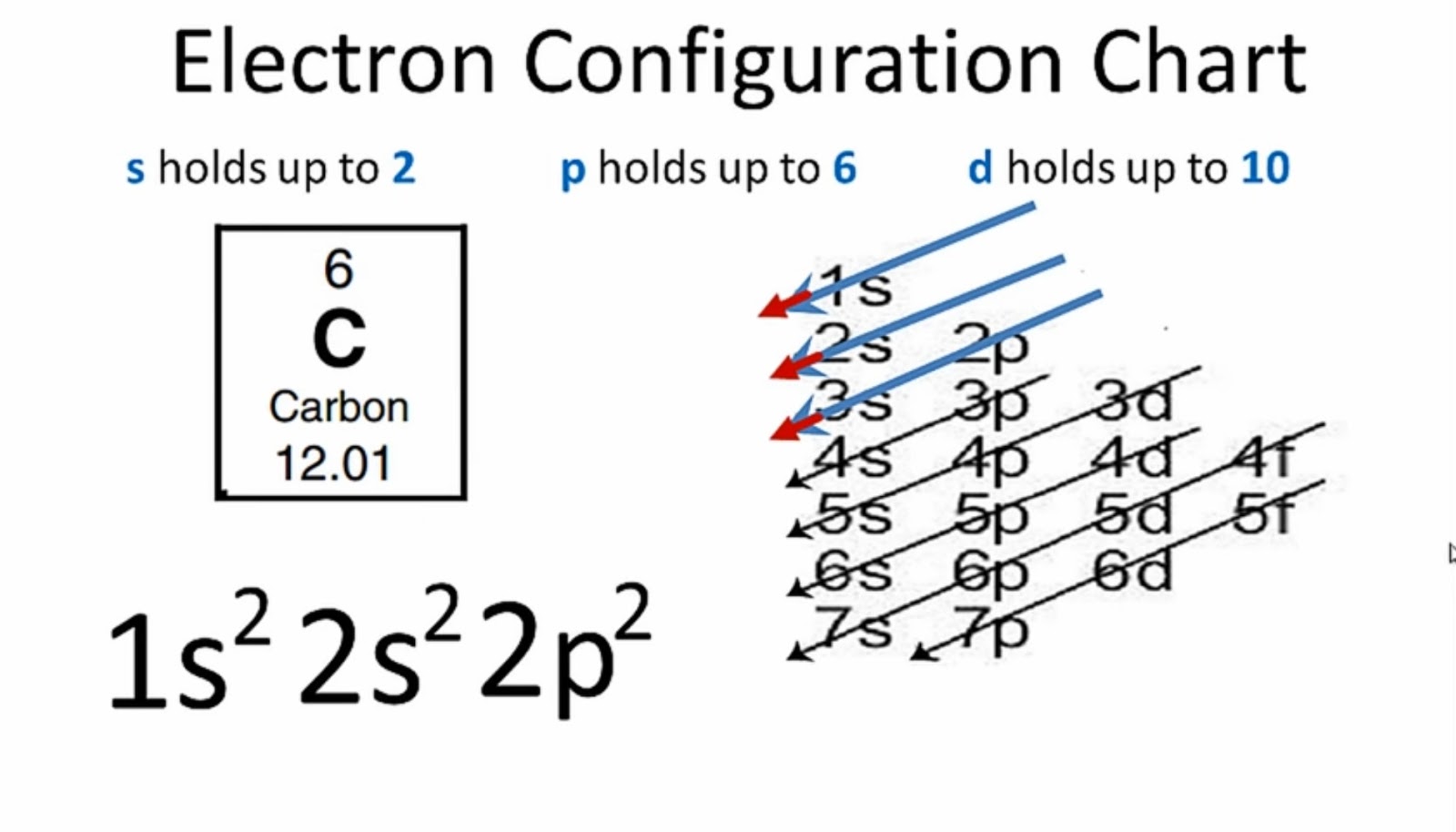
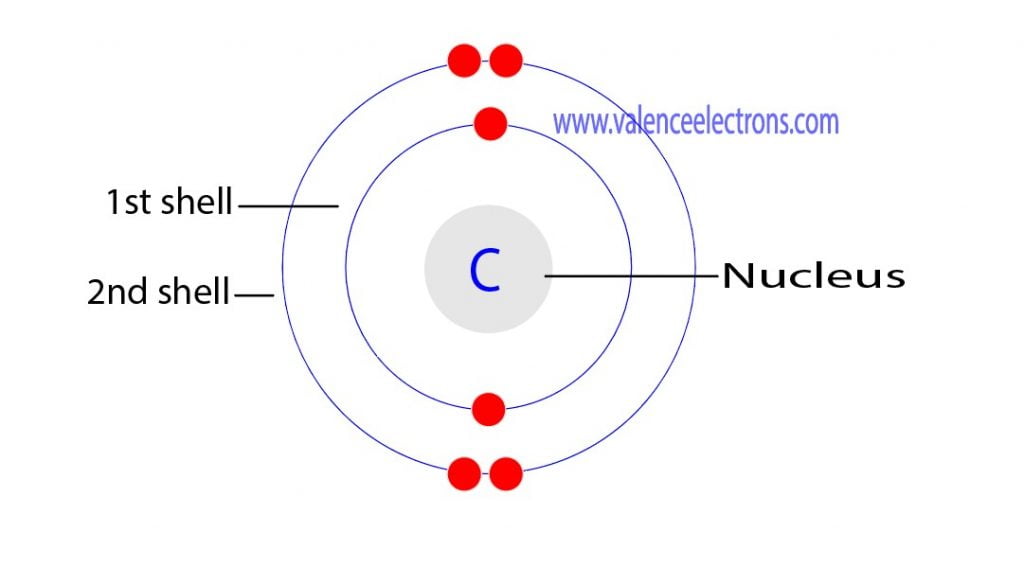
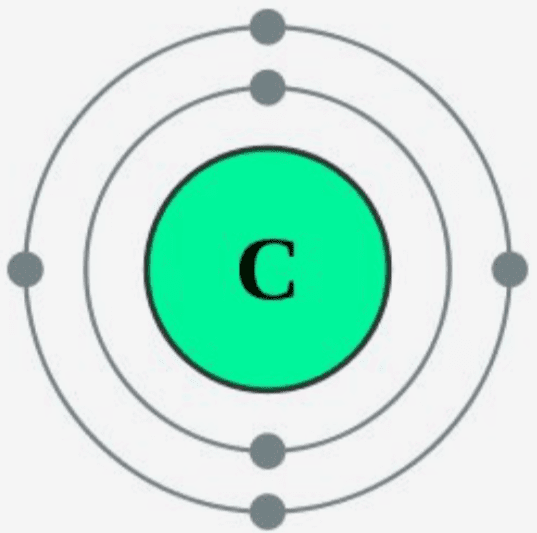
Step-2: Need to do electron configuration of carbon Step 2 is very important. In this step, the electrons of carbon have to be arranged. We know that carbon atoms have a total of six electrons. The electron configuration of carbon shows that there are two electrons in the K shell and four in the L shell.
How to Find the Valence Electrons for Carbon(C)?
Carbon has an atomic number of six (meaning six protons, and six electrons as well in a neutral atom), so the first two electrons fill the inner shell and the remaining four are left in the second shell, which is the valence (outermost) shell.
How to Find the Valence Electrons for Carbon(C)?
Valence electrons are the electrons present in the outermost shell of an atom. You can easily determine the number of valence electrons an atom can have by looking at its Group in the periodic table. For example, atoms in Groups 1 and 2 have 1 and 2 valence electrons, respectively. Atoms in Groups 13 and 18 have 3 and 8 valence electrons.

Carbon Element With Reaction, Properties, Uses, & Price Periodic Table
Carbon has 4 valence electrons. Each oxygen has 6 valence electrons. The -2 charge means that there are 2 extra electrons. Total: 4 + (3 × 6) + 2 = 24 electrons. The final answer MUST have this number of electrons‼! Step 2) Attach the atoms to each other using single bonds ("draw the skeleton structure").

[Class 10 Chemistry] What is Carbon and its compounds? Teachoo
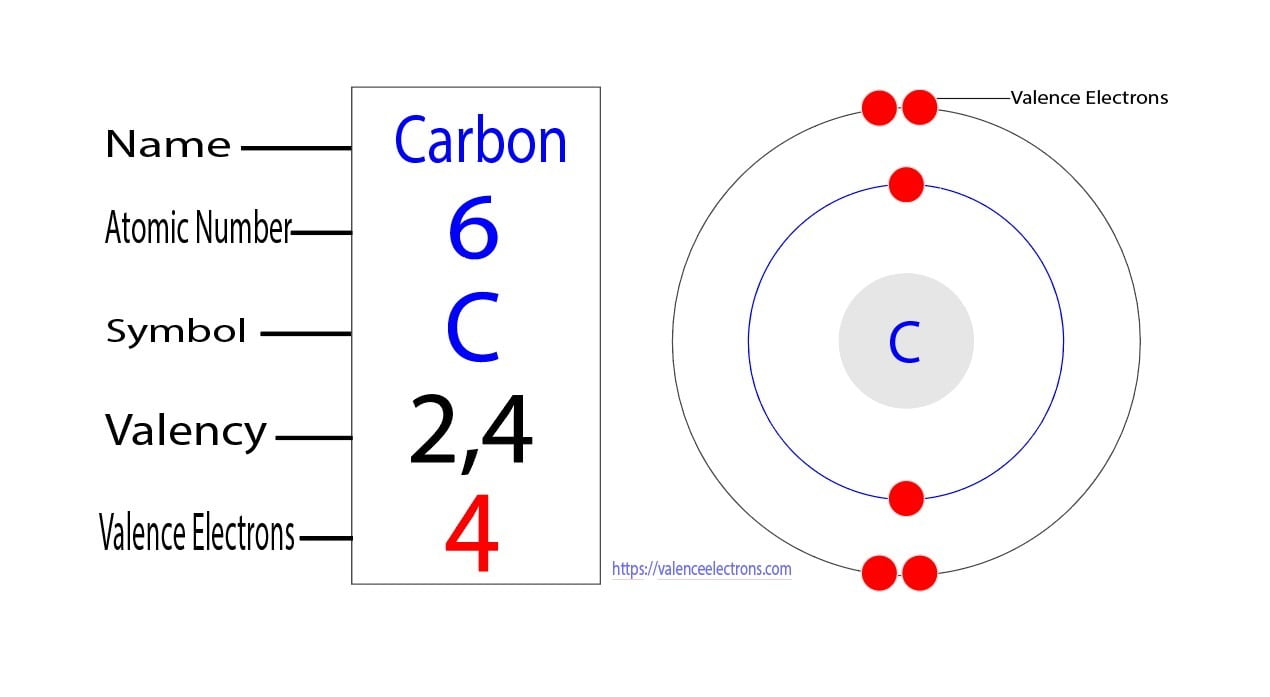
How Many Valence Electrons Does Carbon Have? Carbon has 4 valence electrons in the outermost shell. Carbon has atomic number 6 which belongs to group 14 in the periodic table. A carbon atom has a total of 6 electrons revolving around the nucleus.
What Is the Carbon(C) Electron Configuration?
You can see that in the periodic table, carbon is in group 4, so it has 4 valence electrons. How many valence electrons does oxygen have? 6. Oxygen is in group 16 of the periodic table, so as with the other elements in groups 13-18, you can subtract 10 from the group number to find out the number of valence electrons.

How Many Valence Electrons Does Carbon have? Explain With Different Methods?
What is Valancy of Carbon. According to the octet theory, in order for each element to reach a stable state, the number of electrons it leaves, or gain or mutual shares in order to fill its octet, we will call that element the valence. Thus, the valency of carbon is four.

5 Steps】How Many Valence Electrons Does Carbon Have?Number of Valence Electrons in Carbon
Explanation: To find how many valence electrons are in an atom, you can look at the periodic table: Look at the writing above each Group, or column. The number next to the "A" is the number of valence electrons in an atom of an element in that Group. Carbon is in Group 4A, so it has 4 valence electrons. Answer link
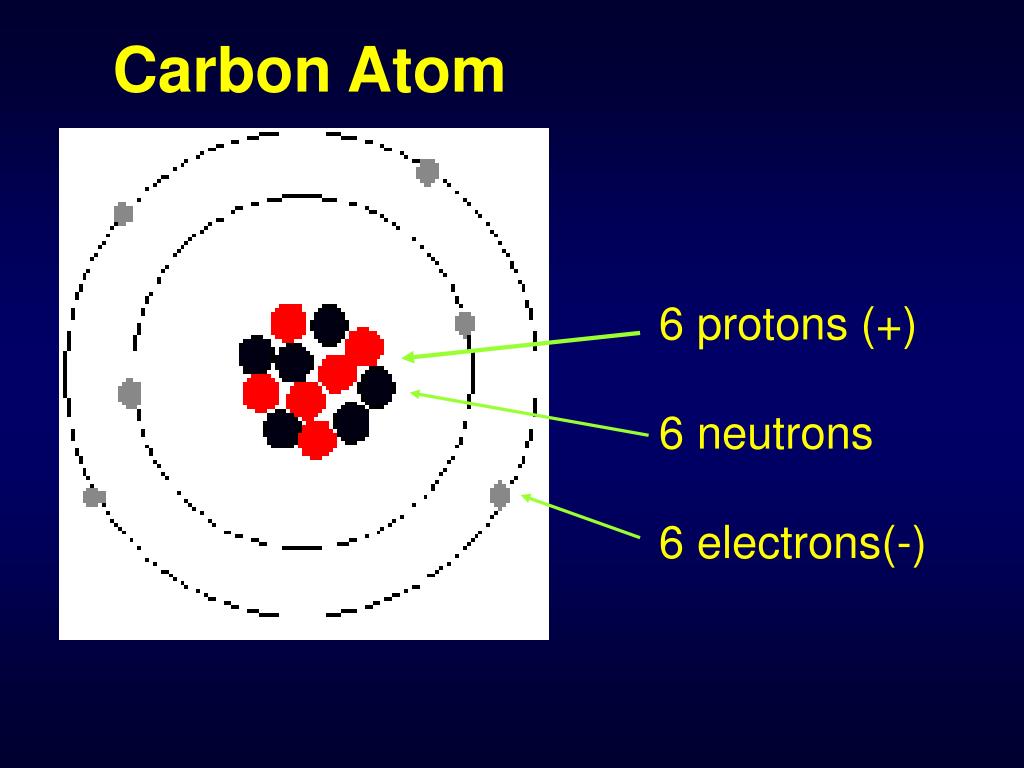
How many protons, neutrons and electrons does carbon have? (2022)
The atomic number of carbon is 6. That is, the number of electrons in carbon is 6. Therefore, a carbon atom will have two electrons in the first shell and four in the 2nd shell. Therefore, the order of the number of electrons in each shell of the carbon(C) atom is 2, 4. Electrons can be arranged correctly through orbits from elements 1 to 18.
How Many Valence Electrons Does Carbon (C) Have? [Valency of Carbon]
The Bohr model shows the atom as a central nucleus containing protons and neutrons, with the electrons in circular electron shells at specific distances from the nucleus, similar to planets orbiting around the sun.
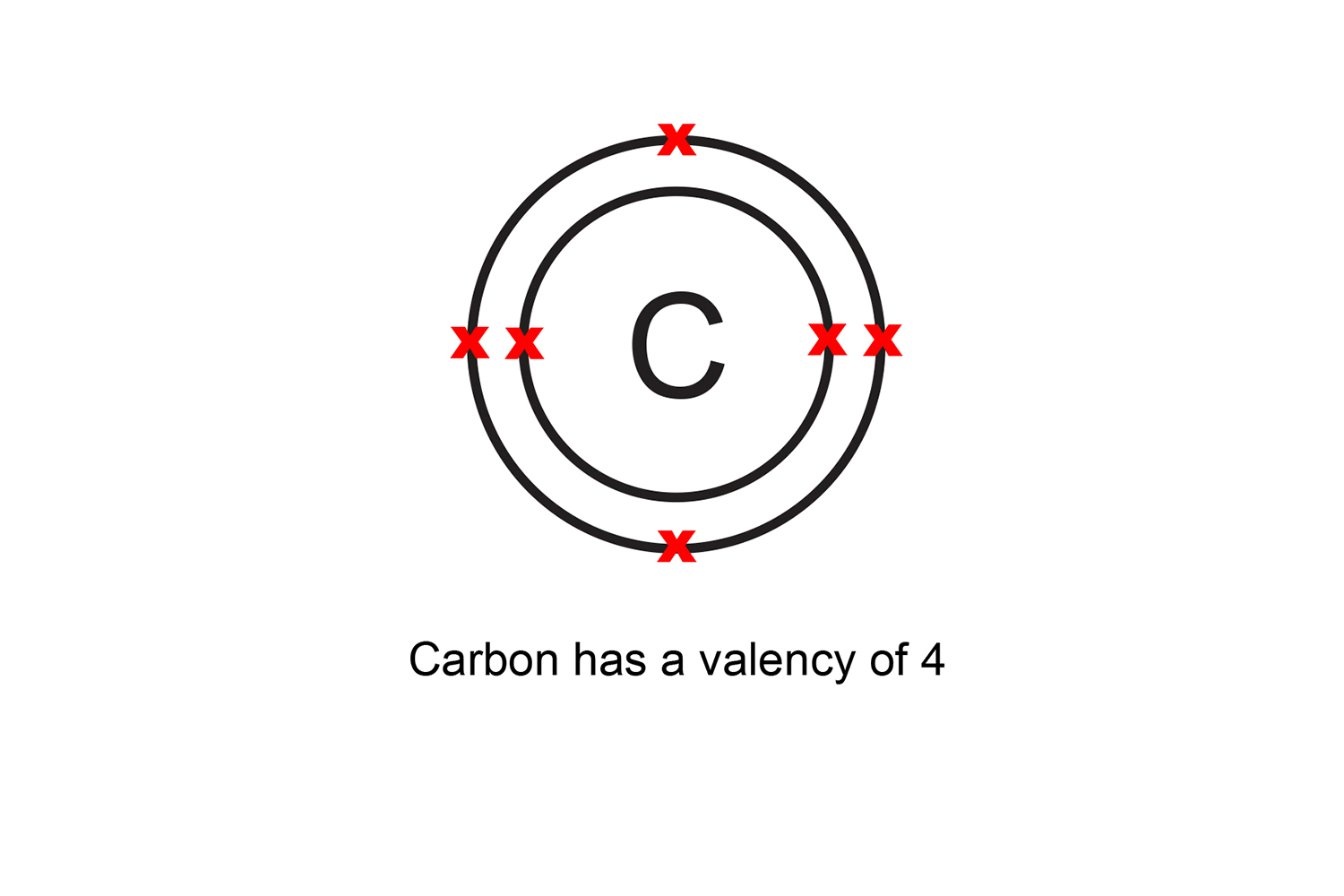
Valency is the number of bonds an atom can make with others
The valence (or valency) of an element is a measure of its combining power with other atoms when it forms chemical compounds or molecules. The concept of valence was developed in the last half of the 19th century and was successful in explaining the molecular structure of many organic compounds. The quest for the underlying causes of valence.
Valence Electrons In Carbon slidesharedocs
Valence electrons are the electrons in the outermost shell, or energy level, of an atom. For example, oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell. We can write the configuration of oxygen's valence electrons as 2s²2p⁴. Created by Sal Khan. Questions.
