
Is CS2 (Carbon disulfide) Ionic or Covalent/Molecular? YouTube
CS2 (Carbon disulfide) is nonpolar due to its linear geometrical shape caused by the presence of no lone pair on the carbon atom.
CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Subscribed 280 62K views 10 years ago CS2 is a linear molecule and the Sulfur (S) atoms on each end are symmetrical. Polarity results from an unequal sharing of valence electrons. Because of this.

Cs2 Polar Or Nonpolar A Comprehensive Guide learnpedia.click
Hey Guys!In this video, we are going to determine the polarity of Carbon Disulfide having a chemical formula of CS2.To know the polarity of this molecule we.

Is CS2 polar or nonpolar? (Carbon Disulfide) YouTube
Carbon disulfide (CS2) is a non-polar molecule. CS2 consists of a carbon (C) atom and two sulfur (S) atoms. The carbon atom is present at the center of the molecule, while both sulfur atoms occupy terminal positions, one on each side, making a linear molecular shape and geometry.

CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
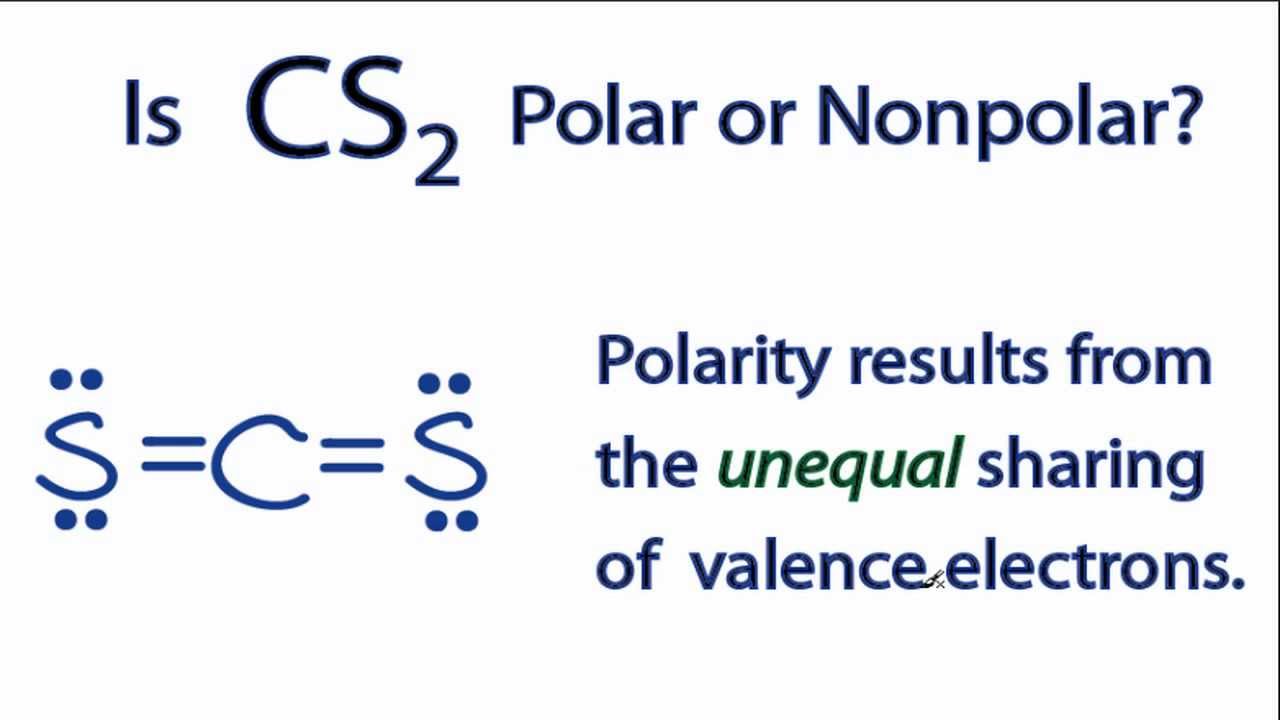
Geometry CS2 Polar or Nonpolar To determine if CS 2 (carbon disulfide) is polar or nonpolar, we need to first determine its geometry. This presumes knowing the rules for drawing a correct Lewis structure and you can find more details about Lewis structures here.
CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
CS2 is a covalent (nonpolar covalent) compound because when one nonmetal combines with another nonmetal, it usually forms a covalent compound. Here, C is a nonmetal and S is also a nonmetal. So when they combine, it forms a covalent compound.

CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Molecular Polarity. To determine if a molecule is polar or nonpolar, it is frequently useful to look at Lewis structures. Nonpolar compounds will be symmetric, meaning all of the sides around the central atom are identical - bonded to the same element with no unshared pairs of electrons.Polar molecules are asymmetric, either containing lone pairs of electrons on a central atom or having atoms.

Is Cs2 Polar Or Nonpolar?
Carbon disulfide (CS2) is a nonpolar, linear molecule. Carbon forms slightly polar bonds with sulfur, but due to the symmetrical arrangement of the bonds, the polarities cancel out. Carbon disulfide is a linear molecule, with carbon at the center. Carbon forms double bonds with each of the sulfur atoms.

Is Cs2 Polar Or Nonpolar Asking List
Lewis Structure Hybridization Molecular geometry Polarity Lewis Structure Lewis structure is the structural representation of the number of valence electrons that participate in the bond formation and nonbonding electron pairs.

Best Overview Is CS2 Polar or Nonpolar? Science Education and Tutorials
C. Explanation of the polar/non-polar nature of CS2 molecule. The CS2 molecule is non-polar due to its symmetrical linear shape and the equal sharing of electrons in its covalent bonds. The carbon-sulfur double bonds are non-polar since both atoms have the same electronegativity value. Additionally, the molecule has no lone pairs of electrons.

CS2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
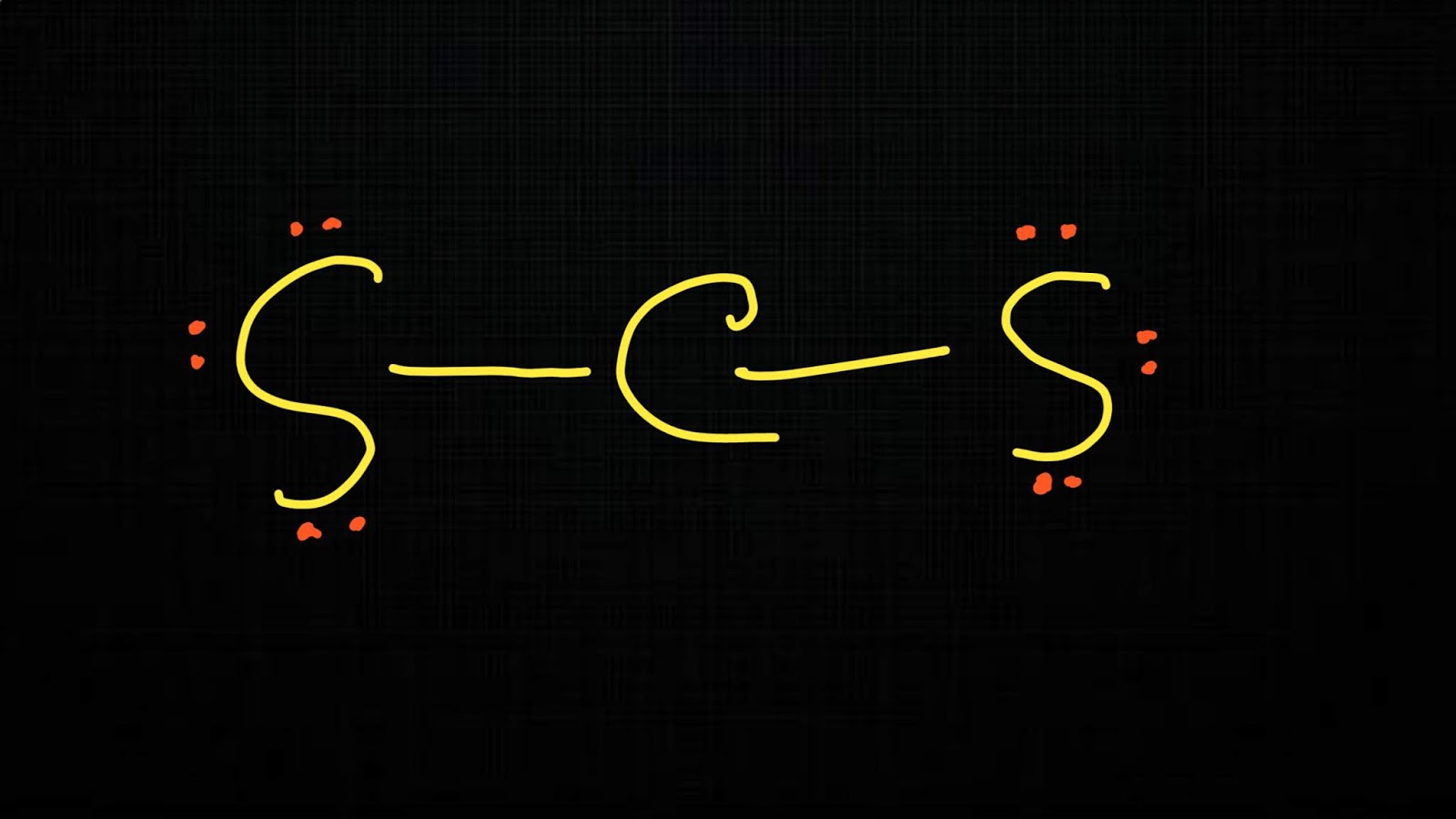
Carbon disulfide (also spelled as carbon disulphide) is an inorganic compound with the chemical formula CS2 and structure S=C=S. It is a colorless, flammable, neurotoxic liquid that is used as a building block in organic synthesis.

Best Overview Is CS2 Polar or Nonpolar? Science Education and Tutorials
A molecule (or polyatomic ion) is polar when one side of the molecule is more positive (or more negative) than the other. This occurs when the polarities of the bonds do not cancel out. For example in CO 2, each carbon-oxygen bond is polar, but CO 2 is a nonpolar molecule .
Solved A. CS2 Is CS2 polar or nonpolar? polar nonpolar ball
CS2 is a NONPOLAR molecule because both the bonds (C=S bonds) are identical and CS2 has symmetrical geometry which cancels out the bond polarity. Let me explain this in detail with the help of CS2 lewis structure and its 3D geometry. Why is CS2 a Nonpolar molecule? (Explained in 2 Steps)

Is CS2 polar or nonpolar? YouTube
Carbon disulfide (CS2) is a flammable, and highly odorous ( not tolerable) molecule in liquid form. It is usually colorless and volatile that can cause a lot of poisonous outcomes. From liver and kidney diseases to weight loss and vision impairment, exposure to CS2 is not desirable.

CS2 Lewis Structure, Hybridization, Polarity and Molecular Shape
Carbon disulfide, or CS2, is a molecule that is not polar. It is nonpolar because its molecular structure is straight, with two sulfur (S) atoms sandwiching a carbon (C) atom. Even though the electronegativity of carbon is 2.55 and that of sulfur is 2.58, the difference is not big enough to make a polar bond.
Is CS2 polar or nonpolar? YouTube
Explain. Question: Is C S 2 a polar or nonpolar molecule? Explain. Polarity of Molecules: In chemistry, molecules are often classified in terms of their polarity: A polar molecule is a molecule.
