
Solved Draw the best Lewis structure for CH2F2. Which of the
CH 2 F 2 Lewis structure CH 2 F 2 (difluoromethane) has one carbon atom, two hydrogen atoms, and two fluorine atoms. In the CH 2 F 2 Lewis structure, there are four single bonds around the carbon atom, with two hydrogen atoms and two fluorine atoms attached to it, and on each fluorine atom, there are three lone pairs.

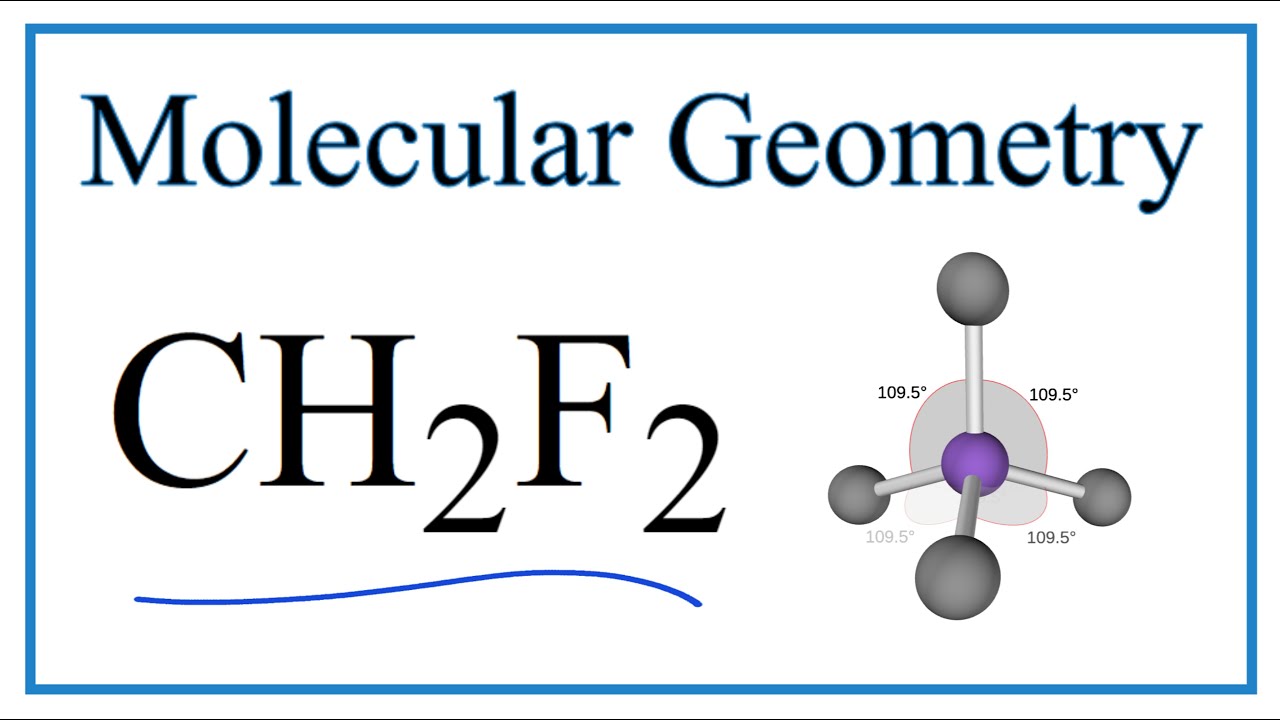
Ch2F2 Molecular Geometry
The N atom has the following Lewis electron dot diagram: It has three unpaired electrons, each of which can make a covalent bond by sharing electrons with an H atom. The electron dot diagram of NH 3 is as follows: Exercise 12.4.2 12.4. 2. Use a Lewis electron dot diagram to show the covalent bonding in PCl 3. Answer.

Solved Draw the best Lewis structure for CH2F2. Which of the
A step-by-step explanation of how to draw the CH2F2 Lewis Dot Structure (Difluoromethane). For the CH2F2 structure use the periodic table to find the total number of valence electro.
Lewis Structure Of Cf2h2 Draw Easy
The first step is to sketch the molecular geometry of the CH2F2 molecule, to calculate the lone pairs of the electron in the central carbon atom; the second step is to calculate the CH2F2 hybridization, and the third step is to give perfect notation for the CH2F2 molecular geometry.

CH2Cl2 Molecular Geometry Science Education and Tutorials
The Lewis structure of XeF 4 indicates six regions of high electron density around the xenon atom: two lone pairs and four bonds: These six regions adopt an octahedral arrangement (Figure \(\PageIndex{6}\)), which is the electron-pair geometry. To minimize repulsions, the lone pairs should be on opposite sides of the central atom (Figure.
Solved Set 5 Data Bond and Molecular Polarity CH4 CH2F2 CF4
The Lewis structure of Difluoromethane will help us understand its structure and atomic constitution. Let us look at some of the properties of Difluoromethane below: Contents CH2F2 Valence Electrons CH2F2 Lewis Structure CH2F2 Hybridization CH2F2 Bond Angles CH2F2 Molecular Geometry Concluding Remarks CH2F2 Valence Electrons
Lewis Dot Structure Ch2f2 💖fluoride Lewis Dot Structure Free Hot Nude
An explanation of the molecular geometry for the CH2F2 (Difluromethane) including a description of the CH2F2 bond angles. The electron geometry for the Diflu.
Ch2f2 Lewis Structure
The first step is to sketch the Lewis structure of the CH2F2 molecule, to add valence electron around the carbon atom; the second step is to add valence electrons to the two fluorine and two hydrogen atoms, and the final step is to combine the step1 and step2 to get the CH2F2 Lewis Structure.

How to draw CH2F2 Lewis Structure? Science Education and Tutorials
For finding the Lewis Structure of CH2F2, we first find o. Hi Guys!This video will help you determine the CH2F2 Lewis structure with a step-by-step procedure. For finding the Lewis Structure of.

CH2F2 Lewis Structure, Molecular Geometry, Hybridization, and Polarity
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".
Molecular Shape Wappingers Central School District
Structure Advanced History Comment on this record 3D Difluoromethane Molecular Formula CH F Average mass 52.023 Da Monoisotopic mass 52.012455 Da ChemSpider ID 6105 More details: Names Properties Searches Spectra Vendors Articles More Names and Synonyms Database ID (s) Validated by Experts, Validated by Users, Non-Validated, Removed by Users

CH2F2 Lewis Structure (Difluoromethane) Lewis, Molecules, Math
Thus far, we have used two-dimensional Lewis structures to represent molecules. However, molecular structure is actually three-dimensional, and it is important to be able to describe molecular bonds in terms of their distances, angles, and relative arrangements in space (Figure 4.14).A bond angle is the angle between any two bonds that include a common atom, usually measured in degrees.

DifluoromethanePolarity Difluoromethane CH2F2 GeometryOfMolecules
Chemical structure: This structure is also available as a 2d Mol file or as a computed 3d SD file The 3d structure may be viewed using Java or Javascript. Fluorocarbon Solutions at Low Termperatures IV. The Liquid Mixtures CH4 + CClF3, CH2F2 + CClF3, CHF3 + CClF3, CF4 + CClF3, C2H6 + CClF3, C2H6 + CF4, and CHF3 + CF4, J. Phys. Chem., 1964.

MakeTheBrainHappy Is CH2F2 Polar or Nonpolar?
CH2F2 lewis structure has a Carbon atom (C) at the center which is surrounded by two Hydrogen atoms (H) and two Fluorine atoms (F). There is a single bond between the Carbon (C) & Fluorine (F) atoms as well as between the Carbon (C) and Hydrogen (H) atoms. There are 3 lone pairs on both the Fluorine atoms (F).

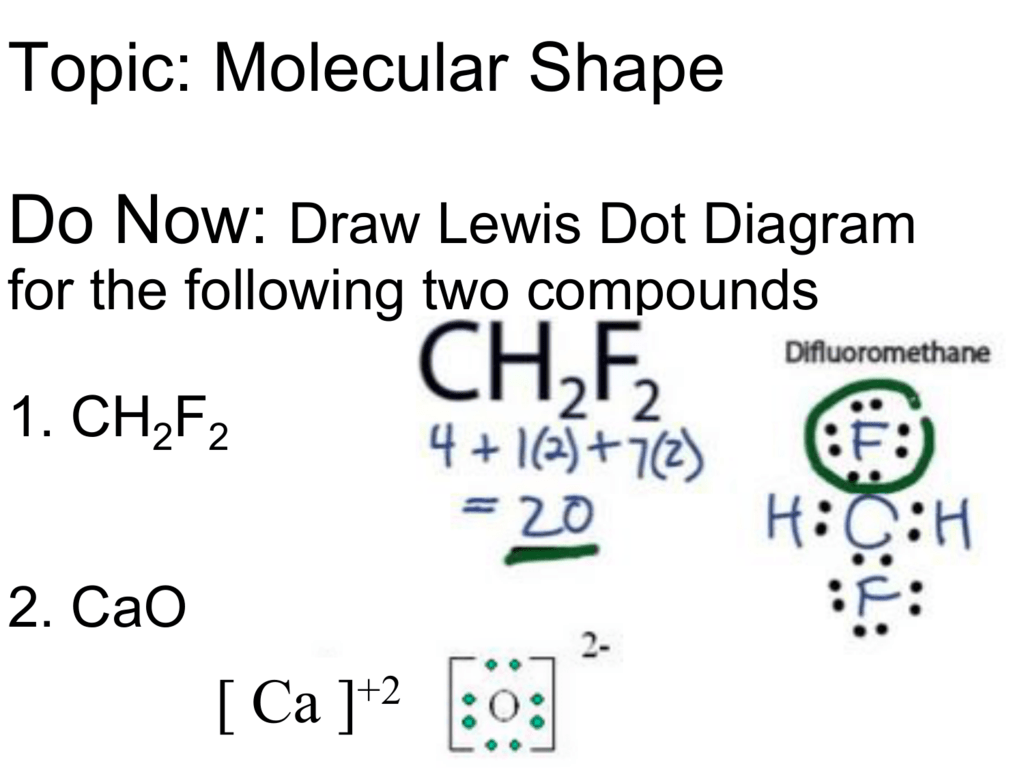
So far, we’ve used 20 of the CH2F2 Lewis structure’s total 20 outermost
CH 2 F 2 Lewis structure is made up of one carbon (C) atom, two hydrogens (H), and two fluorine (F) atoms. The carbon (C) atom is kept at the central position and other atoms are at the surrounding position. The lewis structure of CH 2 F 2 contains 4 single bonds in the form of two C-H bonds and two C-F bonds.
Ch2f2 Lewis Structure
It has the formula of CH 2 F 2. It is a colorless gas in the ambient atmosphere and is slightly soluble in the water, with a high thermal stability. [2] [failed verification] Due to the low melting and boiling point, (-136.0 °C and -51.6 °C respectively) contact with this compound may result in frostbite.
